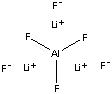| LITHIUM HEXAFLUOROALUMINATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 13821-20-0 |
|
| EINECS NO. | 237-509-4 | |
| FORMULA | Li3AlF6 | |
| MOL WT. | 161.79 | |
|
H.S.CODE |
2826.90 |
|
| TOXICITY | ||
| SYNONYMS |
Lithium cryolite; Aluminate(3-), hexafluoro-, trilithium; |
|
| Trilithium hexafluoroaluminate; Trilithiumhexafluoraluminat (German); Hexafluoroaluminato de trilitio (Spanish); Hexafluoroaluminate de trilithium (French); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white powder |
|
| MELTING POINT | 790 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | 2.75 | |
| SOLUBILITY IN WATER | ||
| pH |
6 |
|
| VAPOR DENSITY | ||
| NFPA RATINGS | ||
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
filler for bonded abrasives; component for welding rods and fluxing agents |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white powder |
|
|
Li3AlF6 |
94.0 - 96.0% | |
|
Fe |
0.02% max | |
| SO4 |
0.02% max | |
|
SiO2 |
0.5% max |
|
|
Cl |
0.01% max |
|
|
Pb |
0.01% max | |
|
WATER |
4.0% max | |
| TRANSPORTATION | ||
| PACKING | 50kgs in bag | |
| HAZARD CLASS | ||
| UN NO. | ||
| DESCRIPTION OF CRYOLITE |
||
|
Cryolite is a white or colorless mineral form of sodium aluminofluoride, which crystallizes in the monoclinic system but has a pseudocubic aspect; found in masses of waxy luster; hardness is 2.5 on Mohs scale, and specific gravity is 3.0. The powder becomes almost invisible in water due to its low refractive index. It is mined in significant quantities in Greenland ( so also known as Greenland spar; ice stone), and in small amounts in elsewhere. Synthetic cryolite is manufactured from hydrofluoric acid, sodium carbonate, and aluminium. Synthetic cryolite is used chiefly as a flux in the electrolytic production of aluminum from bauxite as it effectively lowers down the melting point of alumina. It is used in the glass and enamel industries, in bonded abrasives as a filler, in making salts of sodium and aluminum and porcelaneous glass and in the manufacture of insecticides. Cryolite is a relatively safe fruit and vegetable insecticide. The fluoride ion inhibits many enzymes that contain iron, calcium, and magnesium. Several of these enzymes are involved in energy production in cells, as in the case of phosphatases and phosphorylases. |
||